 |
PROMETRIUM® (progesterone, USP) Capsules contain micronized progesterone for oral administration. Progesterone has a molecular weight of 314.47 and an empirical formula of C 21 H 30 O 2 . Progesterone (pregn-4-ene-3, 20-dione) is a white or creamy white, odorless, crystalline powder practically insoluble in water, soluble in alcohol, acetone and dioxane and sparingly soluble in vegetable oils, stable in air, melting between 126° and 131°C. The structural formula is:
 |
Progesterone is synthesized from a starting material from a plant source and is chemically identical to progesterone of human ovarian origin. PROMETRIUM Capsules are available in multiple strengths to afford dosage flexibility for optimum management. PROMETRIUM Capsules contain 100 mg or 200 mg micronized progesterone.
The inactive ingredients for PROMETRIUM Capsules 100 mg include: peanut oil NF, gelatin NF, glycerin USP, lecithin NF, titanium dioxide USP, D&C Yellow No. 10, and FD&C Red No. 40.
The inactive ingredients for PROMETRIUM Capsules 200 mg include: peanut oil NF, gelatin NF, glycerin USP, lecithin NF, titanium dioxide USP, D&C Yellow No. 10, and FD&C Yellow No. 6.
PROMETRIUM Capsules are an oral dosage form of micronized progesterone which is chemically identical to progesterone of ovarian origin. The oral bioavailability of progesterone is increased through micronization.
After oral administration of progesterone as a micronized soft gelatin capsule formulation, maximum serum concentrations were attained within 3 hours. The absolute bioavailability of micronized progesterone is not known. Table 1 summarizes the mean pharmacokinetic parameters in postmenopausal women after five oral daily doses of PROMETRIUM Capsules 100 mg as a micronized soft-gelatin capsule formulation.
|
||||||||||||||||||||||||
Serum progesterone concentrations appeared linear and dose proportional following multiple dose administration of PROMETRIUM Capsules 100 mg over the dose range 100 mg/day to 300 mg/day in postmenopausal women. Although doses greater than 300 mg/day were not studied in females, serum concentrations from a study in male volunteers appeared linear and dose proportional between 100 mg/day and 400 mg/day. The pharmacokinetic parameters in male volunteers were generally consistent with those seen in postmenopausal women.
Progesterone is approximately 96%-99% bound to serum proteins, primarily to serum albumin (50%-54%) and transcortin (43%-48%).
Progesterone is metabolized primarily by the liver largely to pregnanediols and pregnanolones. Pregnanediols and pregnanolones are conjugated in the liver to glucuronide and sulfate metabolites. Progesterone metabolites which are excreted in the bile may be deconjugated and may be further metabolized in the gut via reduction, dehydroxylation, and epimerization.
The glucuronide and sulfate conjugates of pregnanediol and pregnanolone are excreted in the bile and urine. Progesterone metabolites which are excreted in the bile may undergo enterohepatic recycling or may be excreted in the feces.
The pharmacokinetics of PROMETRIUM Capsules have not been assessed in low body weight or obese patients.
Race:
There is insufficient information available from trials conducted with PROMETRIUM Capsules to compare progesterone pharmacokinetics in different racial groups.
No formal studies have evaluated the effect of hepatic disease on the disposition of progesterone. However, since progesterone is metabolized by the liver, use in patients with severe liver dysfunction or disease is contraindicated (see CONTRAINDICATIONS ). If treatment with progesterone is indicated in patients with mild to moderate hepatic dysfunction, these patients should be monitored carefully.
No formal studies have evaluated the effect of renal disease on the disposition of progesterone. Since progesterone metabolites are eliminated mainly by the kidneys, PROMETRIUM Capsules should be used with caution and only with careful monitoring in patients with renal dysfunction. (see PRECAUTIONS )
Concomitant food ingestion increased the bioavailability of PROMETRIUM Capsules relative to a fasting state when administered to postmenopausal women at a dose of 200 mg.
The metabolism of progesterone by human liver microsomes was inhibited by ketoconazole (IC 50 <0.1 µM). Ketoconazole is a known inhibitor of cytochrome P450 3A4, hence these data suggest that ketoconazole or other known inhibitors of this enzyme may increase the bioavailability of progesterone. The clinical relevance of the in vitro findings is unknown.
Coadministration of conjugated estrogens and PROMETRIUM Capsules to 29 postmenopausal women over a 12 day period resulted in an increase in total estrone concentrations (Cmax 3.68 ng/ml to 4.93 ng/ml) and total equilin concentrations (Cmax 2.27 ng/ml to 3.22 ng/ml) and a decrease in circulating 17(beta) estradiol concentrations (Cmax 0.037 ng/ml to 0.030 ng/ml). The half-life of the conjugated estrogens was similar with coadministration of PROMETRIUM Capsules. Table 2 summarizes the pharmacokinetic parameters.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In a randomized double-blind clinical trial, 358 postmenopausal women, each with an intact uterus, received treatment for up to 36 months. The treatment groups were: PROMETRIUM Capsules at the dose of 200 mg/day for 12 days per 28 day cycle in combination with conjugated estrogens 0.625 mg/day (n=120); conjugated estrogens 0.625 mg/day only (n=119); or placebo (n=119). The subjects in all three treatment groups were primarily Caucasian women (87% or more of each group). The results for the incidence of endometrial hyperplasia in women receiving up to 3 years of treatment are shown in Table 3. A comparison of the PROMETRIUM Capsules plus conjugated estrogens treatment group to the conjugated estrogens only group showed a significantly lower rate of hyperplasia (6% combination product vs. 64% estrogen alone) in the PROMETRIUM Capsules plus conjugated estrogens treatment group throughout 36 months of treatment.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
The times to diagnosis of endometrial hyperplasia over 36 months of treatment are shown in Figure 1. This figure illustrates graphically that the proportion of patients with hyperplasia was significantly greater for the conjugated estrogens group (64%) compared to the conjugated estrogens plus PROMETRIUM Capsules group (6%).
 |
The discontinuation rates due to hyperplasia over the 36 months of treatment are as shown in Table 4. For any degree of hyperplasia, the discontinuation rate for patients who received conjugated estrogens plus PROMETRIUM Capsules was similar to that of the placebo only group, while the discontinuation rate for patients who received conjugated estrogens alone was significantly higher. Women who permanently discontinued treatment due to hyperplasia were similar in demographics to the overall study population.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In the same three year clinical trial, postmenopausal women were treated with PROMETRIUM Capsules in combination with conjugated estrogens, conjugated estrogens only, or placebo. There was no statistically significant difference between the PROMETRIUM Capsules plus conjugated estrogens group and the conjugated estrogens only group in increases of HDL-C and triglycerides, or in decreases of LDL-C. The changes observed in lipid profiles are shown in Table 5.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
In a single-center, randomized, double-blind clinical study that included premenopausal women with secondary amenorrhea for at least 90 days, administration of 10 days of PROMETRIUM Capsules therapy resulted in 80% of women experiencing withdrawal bleeding within 7 days of the last dose of PROMETRIUM Capsules, 300 mg/day (n=20), compared to 10% of women experiencing withdrawal bleeding in the placebo group (n=21).
The rate of secretory transformation was evaluated in a multicenter, randomized, double-blind clinical study in estrogen-primed postmenopausal women. PROMETRIUM Capsules administered orally for 10 days at 400 mg/day (n=22) induced complete secretory changes in the endometrium in 45% of women compared to 0% in the placebo group (n=23).
PROMETRIUM Capsules are indicated for use in the prevention of endometrial hyperplasia in non-hysterectomized postmenopausal women who are receiving conjugated estrogens tablets. They are also indicated for use in secondary amenorrhea.
See accompanying Patient Insert.
General: This product contains peanut oil and should not be used if you are allergic to peanuts.
The following laboratory results may be altered by the use of estrogen-progestin combination drugs:
Increased sulfobromophthalein retention and other hepatic function tests.
Coagulation tests: increase in prothrombin factors VII, VIII, IX and X.
Thyroid function: increase in PBI, and butanol extractable protein bound iodine and decrease in T3 uptake values.
Fasting and 2-hour plasma insulin and glucose levels following an oral glucose tolerance test (OGTT) and fibrinogen levels were measured in patients receiving PROMETRIUM Capsules at a dose of 200 mg/day for 12 days per 28 day cycle in combination with conjugated estrogens 0.625 mg/day (n=120). Table 6 summarizes this data. Plasma insulin levels 2 hours post-OGTT were decreased from baseline. The fasting plasma glucose and fasting plasma insulin levels were also decreased from baseline. Glucose levels 2 hours post-OGTT were increased slightly. There was no effect on fibrinogen levels.
For information on changes in lipid profile, see the Clinical Studies subsection, Table 5.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Progesterone has not been tested for carcinogenicity in animals by the oral route of administration. When implanted into female mice, progesterone produced mammary carcinomas, ovarian granulosa cell tumors and endometrial stromal sarcomas (1). In dogs, long-term intramuscular injections produced nodular hyperplasia and benign and malignant mammary tumors (2). Subcutaneous or intramuscular injections of progesterone decreased the latency period and increased the incidence of mammary tumors in rats previously treated with a chemical carcinogen (3).
Progesterone did not show evidence of genotoxicity in in vitro studies for point mutations or for chromosomal damage. In vivo studies for chromosome damage have yielded positive results in mice at oral doses of 1000 mg/kg and 2000 mg/kg (4). Exogenously administered progesterone has been shown to inhibit ovulation in a number of species and it is expected that high doses given for an extended duration would impair fertility until the cessation of treatment.
Reproductive studies have been performed in mice at doses up to 9 times the human oral dose (5, 6), in rats at doses up to 44 times the human oral dose (7, 8), in rabbits at a dose of 10 µg/day delivered locally within the uterus by an implanted device (9), in guinea pigs at doses of approximately one-half the human oral dose (10) and in rhesus monkeys (11) at doses approximately the human dose, all based on body surface area, and have revealed little or no evidence of impaired fertility or harm to the fetus due to progesterone.
Several studies in women exposed to progesterone have not demonstrated any significant increase in fetal malformations (12). A single case of cleft palate was observed in the child of a woman using PROMETRIUM Capsules in early pregnancy, although definitive causality has not been established. Rare instances of fetal death have been reported in pregnant women prescribed PROMETRIUM Capsules for unapproved indications. Because the studies in humans cannot rule out the possibility of harm, PROMETRIUM Capsules should be used during pregnancy only if indicated (see CONTRAINDICATIONS ).
The administration of any drug to nursing mothers should be done only when clearly necessary since many drugs are excreted in human milk. Detectable amounts of progestin have been identified in the milk of nursing mothers receiving progestins. The effect of this on the nursing infant has not been determined.
The safety and effectiveness of PROMETRIUM Capsules in pediatric patients have not been established.
Table 7 lists adverse experiences which were reported in >/=2% of patients (regardless of relationship to treatment) who received cyclic PROMETRIUM Capsules, 200 mg daily (12 days per calendar month cycle) with daily 0.625 mg conjugated estrogen, in a multicenter, randomized, double-blind, placebo-controlled clinical trial in 875 postmenopausal women.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 8 lists adverse experiences which were reported in >/=5% of patients receiving PROMETRIUM Capsules, 400 mg/day, in a multicenter, randomized, double-blind, placebo-controlled clinical trial in estrogen-primed (6 weeks) postmenopausal women receiving conjugated estrogens 0.625 mg/day and cyclic (10 days per calendar month cycle) PROMETRIUM Capsules at a dose of 400 mg/day, for three cycles.
|
||||||||||||||||||||||||||||||||||||||||||||||||
The most common adverse experiences reported in >/=5% of patients in all PROMETRIUM Capsules dosage groups studied in this trial (100 mg/day to 400 mg/day) were: dizziness (16%), breast pain (11%), headache (10%), abdominal pain (10%), fatigue (9%), viral infection (7%), abdominal distention (6%), musculoskeletal pain (6%), emotional lability (6%), irritability (5%), and upper respiratory tract infection (5%).
Other adverse events reported in <5% of patients taking PROMETRIUM Capsules include:
Autonomic Nervous System Disorders: dry mouth
Body As A Whole: accidental injury, chest pain, fever
Cardiovascular System Disorders: hypertension
Central and Peripheral Nervous System Disorders: confusion, somnolence, speech disorder
Gastrointestinal System Disorders: constipation, dyspepsia, gastroenteritis, hemorrhagic rectum, hiatus hernia, vomiting
Hearing and Vestibular Disorders: earache
Heart Rate and Rhythm Disorders: palpitation
Metabolic and Nutritional Disorders: edema, edema peripheral
Musculoskeletal System Disorders: arthritis, leg cramps, hypertonia, muscle disorder, myalgia
Myo/Endo/Pericardial and Valve Disorders: angina pectoris
Psychiatric Disorders: anxiety, impaired concentration, insomnia, personality disorder
Reproductive System Disorders: leukorrhea, uterine fibroid, vaginal dryness, fungal vaginitis, vaginitis
Resistance Mechanism Disorders: abscess, herpes simplex
Respiratory System Disorders: bronchitis, nasal congestion, pharyngitis, pneumonitis, sinusitis
Skin and Appendages Disorders: acne, verruca, wound debridement
Urinary System Disorders: urinary tract infection
Vision Disorders: abnormal vision
White Cell and Resistance Disorders: lymphadenopathy
The following adverse experiences have been reported with PROMETRIUM Capsules in other U.S. clinical trials: increased sweating, asthenia, tooth disorder, anorexia, increased appetite, nervousness, and breast enlargement.
The following spontaneous adverse events have been reported during the foreign marketing of PROMETRIUM Capsules: reversible cases of hepatitis and elevated transaminases. These events occurred mainly in patients receiving high doses of up to 1200 mg.
The following additional adverse experiences have been observed in women taking progestins in general: breakthrough bleeding, spotting, change in menstrual flow, amenorrhea, changes in weight (increase or decrease), changes in the cervical squamo-columnar junction and cervical secretions, cholestatic jaundice, anaphylactoid reactions and anaphylaxis, rash (allergic) with and without pruritus, melasma or chloasma, pyrexia, and insomnia.
No studies on overdosage have been conducted in humans. In the case of overdosage, PROMETRIUM Capsules should be discontinued, and the patient should be treated symptomatically.
Prevention of endometrial hyperplasia --PROMETRIUM Capsules should be given as a single daily dose in the evening, 200 mg orally for 12 days sequentially per 28 day cycle, to postmenopausal women with a uterus who are receiving daily conjugated estrogens tablets.
Secondary Amenorrhea --PROMETRIUM Capsules may be given as a single daily dose of 400 mg in the evening for 10 days.
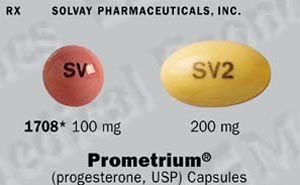
PROMETRIUM® (progesterone, USP) Capsules 100 mg are round, peach-colored capsules branded with black imprint "SV", available in bottles of 100 capsules (NDC0032-1708-01).
PROMETRIUM® (progesterone, USP) Capsules 200 mg are oval, pale yellow- colored capsules branded with black imprint "SV2", available in bottles of 100 capsules (NDC0032-1711-01).
Store at 25°C (77°F). Excursions permitted to 15-30°C (59-86°F).
Dispense in tight, light-resistant container as defined in USP/NF, accompanied by a Patient Insert.
Protect from excessive moisture.
Rx only
Manufactured by: R. P. Scherer North America, St. Petersburg, FL 33716
Marketed by: Solvay Pharmaceuticals, Inc., Marietta, GA 30062.
Copyright© 1999 Solvay Pharmaceuticals, Inc.
All rights reserved.
9563 3E Rev 9/99
 |